The Structure of Atoms
To understand spectral lines you have to understand some Atomic Physics.
- What makes an element?
The number of protons in the nucleus of an atom is what
defines an atom.
- For example: Hydrogen (H) atoms have 1 proton (p+).
- H with p+ and 1 electron (e-) is
"neutral" hydrogen (1H1).
- H with p+ and e+ and a neutron
(n0) is a "heavy" isotope of Hydrogen called deuterium
(2H1).
- If a p+ is added to Hydrogen, we then have a different
element - Helium (4He2).

Q. How many neutrons in 238U92?
Looks like a total of 238 nucleons, and 92 p+. So, it must
have 238 - 92 = 146 n0.
- What does this have to do with light and spectral lines?
Lots of clever experiments around the turn of the century
demonstrated that :
- Light can be thought of as a stream of "quanta" called
photons. Each photon carries an energy E = h
(h is "Planck's
constant",
is the frequency).
- Atoms had a crazy structure in which only certain orbits were allowed
for the electrons - that word again, the orbits are "quantized".

- "g" = "ground" state = lowest energy configuration
- "1" = 1st excited state = higher energy configuration
- "2" = 2nd excited state = higher energy yet
- etc. right on up to where the electron is no longer bound to the atom.
Atoms and Spectra
One of Nature's most basic rules:
Systems naturally seek their lowest available energy state - or - logs
roll downhill
- So, left alone, hydrogen atoms tend to be in the ground state.
Now, let's bombard an H atom with photons. What happens? Most
of the photons go zipping right past without interacting with the
H atom. But! photons with just the RIGHT energy get absorbed by the
atom
Right means that the energy of the photon corresponds to the
energy level difference between "allowed" orbits in the H atom.
Absorbed means that the energy of the photon will now be gone
and the atom will be in a higher energy state.

- A photon with frequency
will be absorbed by an atom if the energy of the photon
corresponds to an energy level difference between allowed states in
the atom
- What happens next? Remember, Nature seeks the lowest available
energy level so the e- bumped to an excited orbit will drop
back to the ground state.
- Another Law of Nature, Conservation of Energy , states
that the energy difference between the excited state and ground state
must appear somewhere when the atom makes the transition. "Somewhere"
is as a photon with the energy of the original one.

- Below is a schematic diagram of the allowed orbits in a Hydrogen
atom. If you can answer the questions about it, you've got the idea.
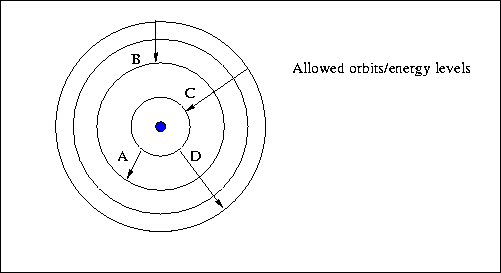
- Which transition(s) correspond(s) to the absorption
of a photon?
- Which transition corresponds to the highest energy
photon emitted ?
- Which transition corresponds to the shortest wavelength
photon emitted?
- Which transition corresponds to the highest frequency
photon emitted?




